Time:2024-03-28
All the sudden emergence is actually a premeditated mutual encouragement! Borrowing words from Chinese writer Han Han: “In this world, there is no unreasonable thing that emerges. Without a lot of accumulation and thinking, things cannot be done well. People can skip school, but they must learn. There are too many capable people in this world, and the limits you think may not be the starting point for others, so only by constantly striving can you not be ashamed.”


About QCS R&D Center
QCS (Quality Control Solutions) Reference Material R&D Center is a high-tech enterprise under Shenzhen ChemSrong, specializing in the R&D, production, testing, and assignment of reference materials.
The R&D center is located in Zhongcheng Life Science Park, Pingshan District, Shenzhen. It covers an area of 3000 square meters and is equipped with 100 sets of high-end instruments and equipment. It currently has more than 90 employees, including 4 professional teams led by PhDs. More than 30% of the team members hold master's degrees or above. Our professional fields span across fields such as organic chemistry, analytical chemistry, pharmacology, pharmacokinetics, and process research and development. The R&D center consists of three professional technical laboratories: separation and purification laboratory, analysis and testing laboratory, and organic synthesis laboratory, as well as a quality department.
The QCS Reference Material R&D Center can currently provide over 50000 types of standard materials with an annual sales volume of over 100 million yuan. Mainly covering the fields of medicine, agriculture/veterinary medicine, food and environment. The product has provided high-quality services for the analysis and testing work of tens of thousands of enterprises and institutions, with typical users including government agencies, universities and research institutes, production enterprises, etc.
Regarding QCS "Zero Defects" through ISO 17034
ISO 17034 is an international standard issued by the International Organization for Standardization (ISO) for a quality management system for the production of reference materials. It provides requirements and guidelines for the preparation of reference materials to ensure the production of reference materials with traceability, accuracy and repeatability.
We are well aware of the important significance of standard materials as "measuring scales" for users. To standardize the production process of our company's standard materials and ensure the quality of standard material production, the QCS Reference Material R&D Center (QCS) has strictly followed the requirements of the international standard ISO 17034 since its establishment, and has established a comprehensive quality management system and technical platform. Starting from three years ago, we have been preparing for the ISO 17034 certification work. After several years of dedicated exploration, we have established a comprehensive management system suitable for the production of our company's standard materials. This year, we passed the professional evaluation of the ANSI National Accreditation Board (ANBA).
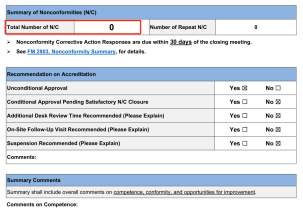
It is rare for the QCS Standard Material Research and Development Center laboratory to pass the first review with a score of
According to ISO/IEC 17025, certified reference materials provided by producers of reference materials that comply with ISO 17034 have metrological traceability. The QCS Reference Material R&D Center has the ability and qualifications to develop and produce standard materials that meet international standards. Customers who use QCS products can obtain more accurate and effective results.
Regarding ISO 17034

About ANAB in the United States
The ANSI National Accreditation Board (ANAB) is a subsidiary of the American National Standards Institute, Inc. (ANSI), engaged in accreditation and evaluation activities for certification agencies, laboratories, inspection agencies, certification and verification agencies, and is the largest multidisciplinary accreditation body in the Western Hemisphere.
ANAB certification originated from the American Quality Association and collaborated with the National Institute of Standards in the United States. Composed of experts from different industries and their representatives of interests, it is a non-profit accredited institution; To provide fair, independent, and efficient certification recognition and certification supervision and management services for certification agencies worldwide. ANAB is a member of the International Accreditation Forum (IAF) and the International Laboratory Accreditation Cooperation (ILAC), which have signed multilateral mutual recognition agreements and hold an irreplaceable important position in the field of international certification and accreditation. The certification certificate issued by its accreditation authority has a high level of authoritative credibility and global mutual recognition effectiveness. The ISO 17034 qualification recognized by ANAB is also recognized by more than 100 other contracting organizations, achieving mutual recognition of global results.
Laboratory ISO certification consulting services
The QCS Reference Material R&D Center has professional and experienced consultants who provide tailored laboratory ISO certification consulting solutions for clients. We can provide certification consulting services for ISO17034 and ISO17025 at affordable prices and thoughtful service. If you need related services, please pay attention to the official account and backend for consultation; Or scan the QR code for ISO certification consultation at the end of the text for communication.
ISO/IEC 17025:2017 Testing/Calibration
ISO17025 certification is an internationally recognized laboratory quality management system certification, mainly aimed at evaluating and certifying the laboratory's technical capabilities, management level, reliability and accuracy of test results, and other aspects. This certification standard aims to ensure that the laboratory's test results are internationally recognized and trusted, and to enhance the laboratory's competitiveness and credibility. ISO17025 certification is not only applicable to independent laboratories, but also to internal laboratories of enterprises, which is of great significance for improving the management level and technical capabilities of laboratories.
Producer approval of ISO/IEC 17034 standard substances
Standard substances/samples are used to calibrate measurement systems, evaluate measurement procedures, and assign values and quality control to other materials. In some cases, standard materials/samples are the "ruler" of measurement in the relevant field and an important medium for achieving metrological traceability. ISO 17034 specifies the general requirements for the production capacity of reference materials, aimed at being part of the general quality assurance program for the production of reference materials. It covers the production requirements for various types of reference materials (including certified reference materials). One of the explanations states that regulatory and certification bodies can also use ISO 17034 to evaluate the capabilities of standard substance producers.
All the sudden emergence is actually a premeditated mutual encouragement! Borrowing words from Chinese writer Han Han: “In this world, there is no unreasonable thing that emerges. Without a lot of accumulation and thinking, things cannot be done well. People can skip school, but they must learn. There are too many capable people in this world, and the limits you think may not be the starting point for others, so only by constantly striving can you not be ashamed.”


About QCS R&D Center
QCS (Quality Control Solutions) Reference Material R&D Center is a high-tech enterprise under Shenzhen ChemSrong, specializing in the R&D, production, testing, and assignment of reference materials.
The R&D center is located in Zhongcheng Life Science Park, Pingshan District, Shenzhen. It covers an area of 3000 square meters and is equipped with 100 sets of high-end instruments and equipment. It currently has more than 90 employees, including 4 professional teams led by PhDs. More than 30% of the team members hold master's degrees or above. Our professional fields span across fields such as organic chemistry, analytical chemistry, pharmacology, pharmacokinetics, and process research and development. The R&D center consists of three professional technical laboratories: separation and purification laboratory, analysis and testing laboratory, and organic synthesis laboratory, as well as a quality department.
The QCS Reference Material R&D Center can currently provide over 50000 types of standard materials with an annual sales volume of over 100 million yuan. Mainly covering the fields of medicine, agriculture/veterinary medicine, food and environment. The product has provided high-quality services for the analysis and testing work of tens of thousands of enterprises and institutions, with typical users including government agencies, universities and research institutes, production enterprises, etc.
Regarding QCS "Zero Defects" through ISO 17034
ISO 17034 is an international standard issued by the International Organization for Standardization (ISO) for a quality management system for the production of reference materials. It provides requirements and guidelines for the preparation of reference materials to ensure the production of reference materials with traceability, accuracy and repeatability.
We are well aware of the important significance of standard materials as "measuring scales" for users. To standardize the production process of our company's standard materials and ensure the quality of standard material production, the QCS Reference Material R&D Center (QCS) has strictly followed the requirements of the international standard ISO 17034 since its establishment, and has established a comprehensive quality management system and technical platform. Starting from three years ago, we have been preparing for the ISO 17034 certification work. After several years of dedicated exploration, we have established a comprehensive management system suitable for the production of our company's standard materials. This year, we passed the professional evaluation of the ANSI National Accreditation Board (ANBA).
It is rare for the QCS Standard Material Research and Development Center laboratory to pass the first review with a score of
According to ISO/IEC 17025, certified reference materials provided by producers of reference materials that comply with ISO 17034 have metrological traceability. The QCS Reference Material R&D Center has the ability and qualifications to develop and produce standard materials that meet international standards. Customers who use QCS products can obtain more accurate and effective results.


Regarding ISO 17034

About ANAB in the United States
The ANSI National Accreditation Board (ANAB) is a subsidiary of the American National Standards Institute, Inc. (ANSI), engaged in accreditation and evaluation activities for certification agencies, laboratories, inspection agencies, certification and verification agencies, and is the largest multidisciplinary accreditation body in the Western Hemisphere.
ANAB certification originated from the American Quality Association and collaborated with the National Institute of Standards in the United States. Composed of experts from different industries and their representatives of interests, it is a non-profit accredited institution; To provide fair, independent, and efficient certification recognition and certification supervision and management services for certification agencies worldwide. ANAB is a member of the International Accreditation Forum (IAF) and the International Laboratory Accreditation Cooperation (ILAC), which have signed multilateral mutual recognition agreements and hold an irreplaceable important position in the field of international certification and accreditation. The certification certificate issued by its accreditation authority has a high level of authoritative credibility and global mutual recognition effectiveness. The ISO 17034 qualification recognized by ANAB is also recognized by more than 100 other contracting organizations, achieving mutual recognition of global results.
Laboratory ISO certification consulting services
The QCS Reference Material R&D Center has professional and experienced consultants who provide tailored laboratory ISO certification consulting solutions for clients. We can provide certification consulting services for ISO17034 and ISO17025 at affordable prices and thoughtful service. If you need related services, please pay attention to the official account and backend for consultation; Or scan the QR code for ISO certification consultation at the end of the text for communication.
ISO/IEC 17025:2017 Testing/Calibration
ISO17025 certification is an internationally recognized laboratory quality management system certification, mainly aimed at evaluating and certifying the laboratory's technical capabilities, management level, reliability and accuracy of test results, and other aspects. This certification standard aims to ensure that the laboratory's test results are internationally recognized and trusted, and to enhance the laboratory's competitiveness and credibility. ISO17025 certification is not only applicable to independent laboratories, but also to internal laboratories of enterprises, which is of great significance for improving the management level and technical capabilities of laboratories.
Producer approval of ISO/IEC 17034 standard substances
Standard substances/samples are used to calibrate measurement systems, evaluate measurement procedures, and assign values and quality control to other materials. In some cases, standard materials/samples are the "ruler" of measurement in the relevant field and an important medium for achieving metrological traceability. ISO 17034 specifies the general requirements for the production capacity of reference materials, aimed at being part of the general quality assurance program for the production of reference materials. It covers the production requirements for various types of reference materials (including certified reference materials). One of the explanations states that regulatory and certification bodies can also use ISO 17034 to evaluate the capabilities of standard substance producers.
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号