Time:2024-03-28
01 Introduction
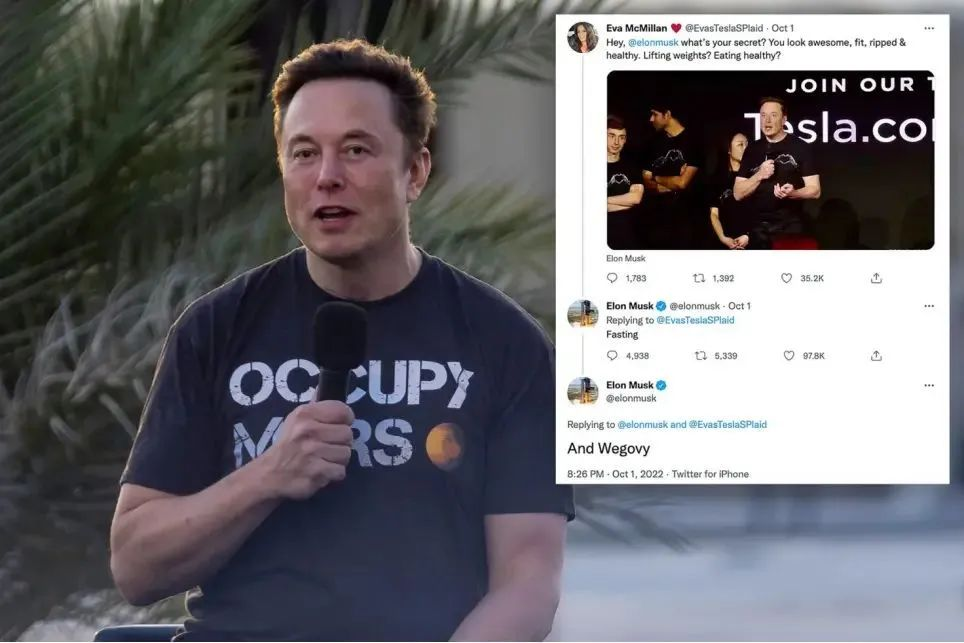
As winter fades away and the arrival of summer approaches, the lightweight clothing fails to conceal the restless human form. Recently, Elon Musk, CEO of Tesla, a company renowned for its innovative transportation solutions, has also joined the ranks of those seeking weight loss through pharmaceutical means. He has taken personally the newly trending slimming drug, Semaglutide.
02 Introduction to Semaglutide
According to the different causes of diabetes, the World Health Organization (WHO) divides diabetes into four types: Type 1 diabetes; Type 2 diabetes; Pregnancy diabetes; Special type diabetes, of which Type 2 diabetes is the most common type. For the treatment of Type 2 diabetes, the main products of new therapeutic drugs in recent years are GLP-1, SGLT-2 inhibitor and DPP4 inhibitor.
SGLT-2 inhibitors and DPP4 inhibitors were relatively launched earlier. In terms of SGLT-2 inhibitors, Dapagliflozin, jointly developed by Bristol-Myers Squibb and AstraZeneca, was launched in the European Union in 2012 and in the United States in 2014. Canagliflozin, which was acquired by Mitsubishi Pharmaceuticals and Johnson&Johnson's subsidiary of Johnson&Johnson, was launched in the United States in 2013. Empagliflozin, which is a partnership between Boehringer Ingelheim and Lilly, was approved by the FDA in 2014; In terms of DPP4 inhibitors, in 2006, Merck's original research of Sitagliptin was launched, Novartis Vildagliptin was launched in 2007, Saxagliptin, jointly developed by Bristol Myers Squibb and AstraZeneca, was launched in 2009, Takeda Alogliptin was launched in 2010, and so on. Although in recent years, new drugs have been continuously approved, such as the first self-developed net drug in China, Hengrui Pharmaceutical Innovation Drug, and SGLT2 inhibitor Henagliflozin, which was launched in 2021. However, GLP-1 drug is undoubtedly the most eye-catching star in recent years in the medical research and development massive free-for-all in the field of type 2 diabetes, and Smeaglutide is undoubtedly one of the trending products.
Semaglutide injection is a hit product of Novo Nordisk. According to Novo Nordisk's financial report, in the first half of 2023, Ozmmpic (trade name for slimming indications of Semaglutide injection) achieved revenue of 41.741 billion Danish kroner (1 Danish kroner is approximately equivalent to 1.05 RMB, QCS editor refers to the exchange rate from February 29, 2024), and Wegovy (trade name for slimming indications of Semaglutide injection) achieved revenue of 12.081 billion Danish kroner, totaling 53.822 billion Danish kroner, equivalent to 7.6 billion US dollars, with an expected total of 20 billion US dollars for the whole year. In the first half of 2023, Novo Nordisk's revenue in China was 8.928 billion Danish kroner, of which Semaglutide Injection (for blood sugar reduction) was 2.213 billion Danish kroner.
03 Independently developed stable isotope product of Semaglutide
Stable isotope markers of Semaglutide can be used for pharmacokinetic, safety, and tolerability studies. Novo Nordisk published two studies in 2018 on the pharmacokinetics, safety, and tolerability of smeglutide in subjects with liver injury or renal insufficiency. This stable isotope marker is used as an internal standard substance for plasma bioanalysis.
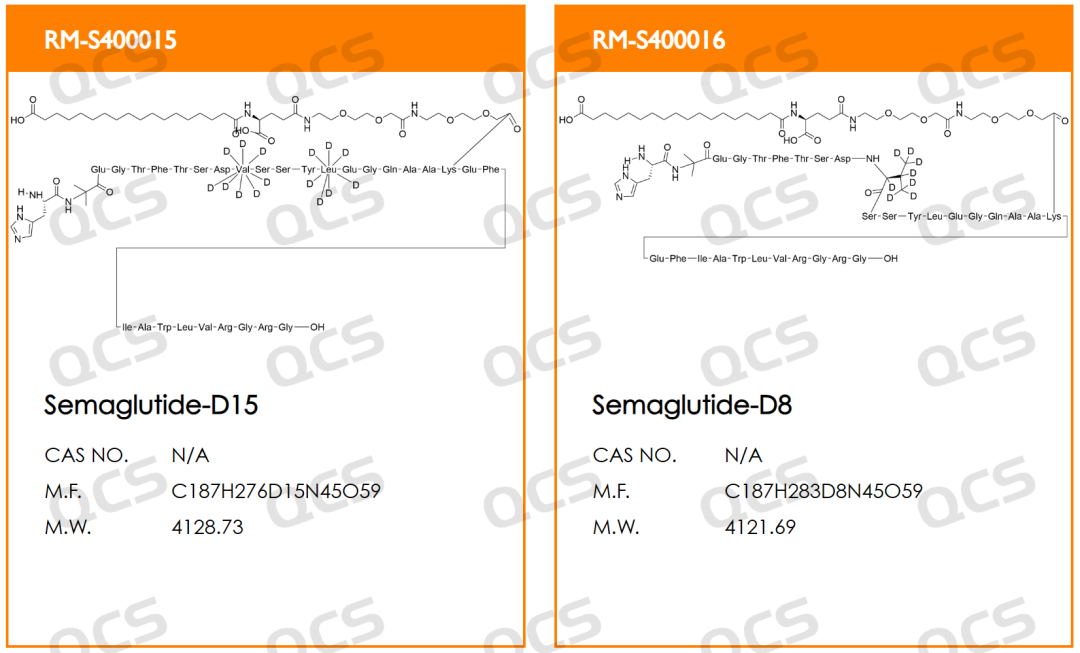
QCS Standard Material R&D Center has developed stable isotope products of Semaglutide-D8 and Semaglutide-D15 (CAT No.: RM-S400016 and RM-S400015) to meet the domestic pharmaceutical research and development needs. These stable isotopes can be applied in the research fields of drug bioavailability, drug distribution, biotransformation and excretion, drug interactions, drug stereoselectivity, etc., which is helpful for related pharmacokinetic studies.


This time, we will use the example of Semaglutide-D8 (CAT No.: RM-S400016), a deuterated valine modified analogue of semaglutide, to introduce QCS drug molecular stable isotope products.
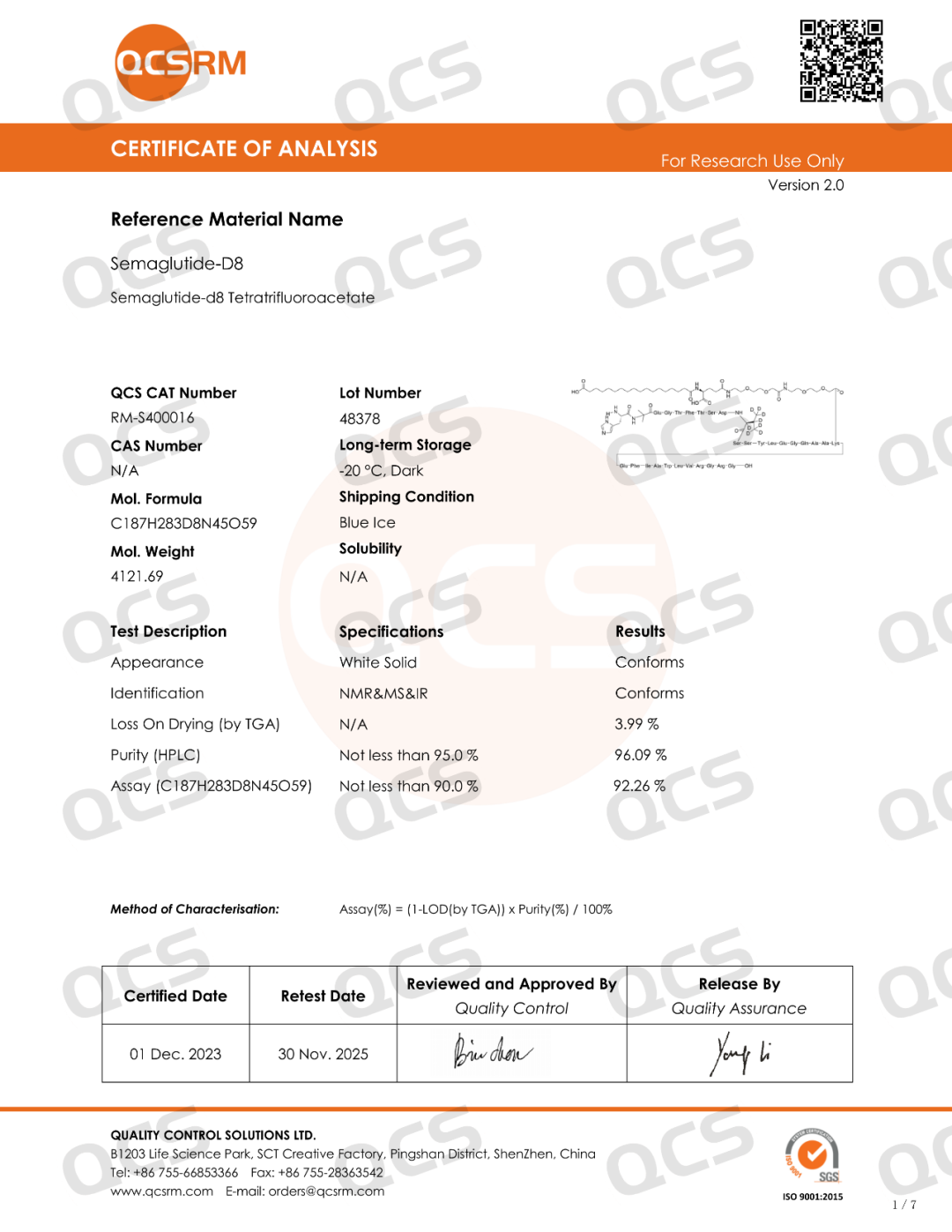
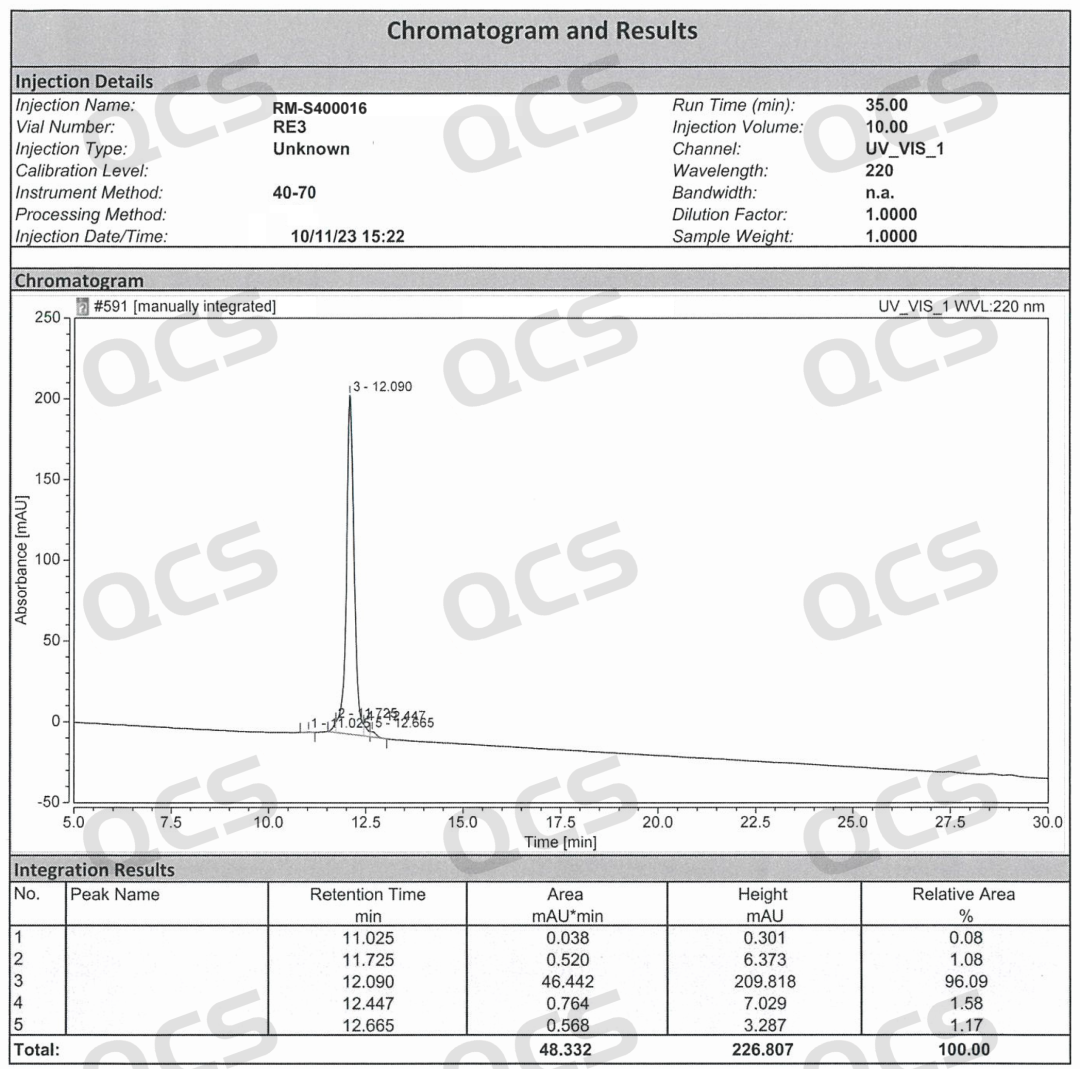
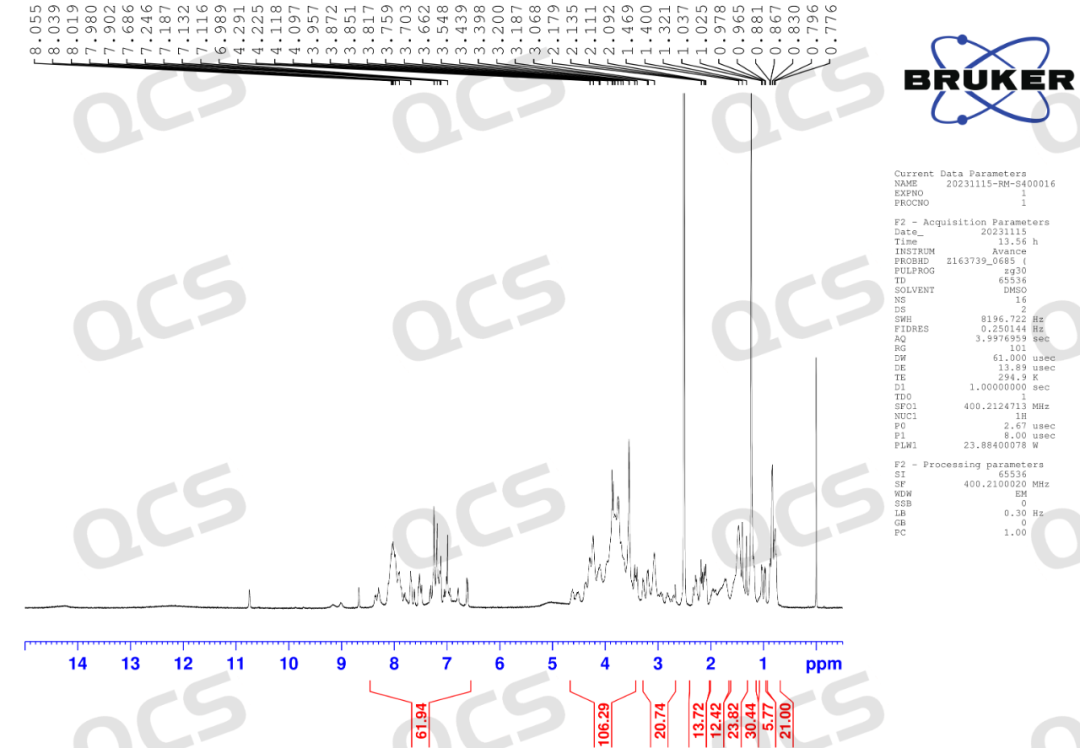
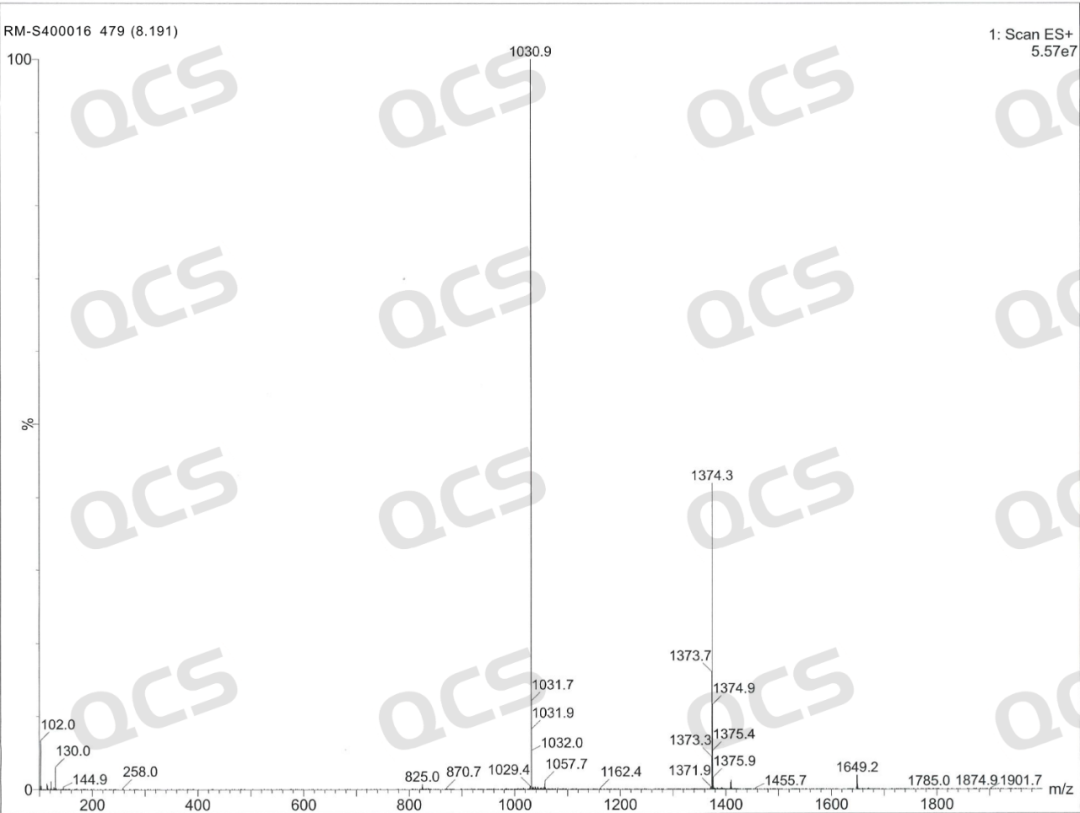
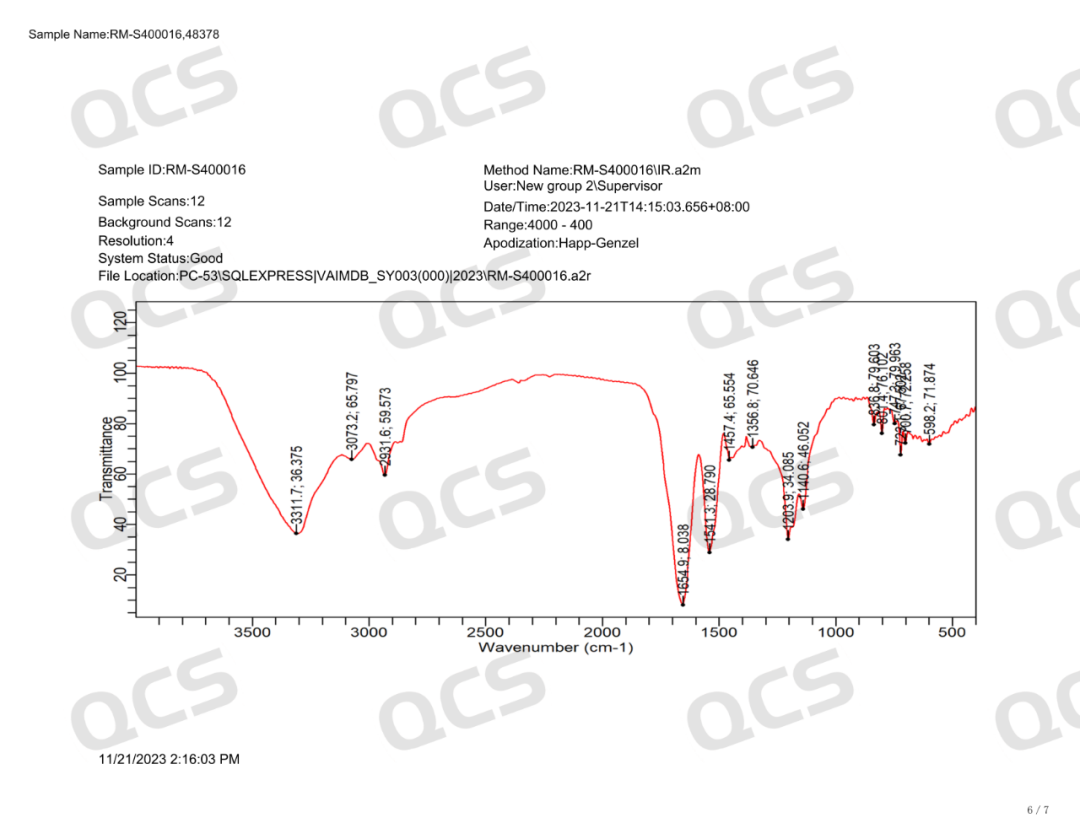
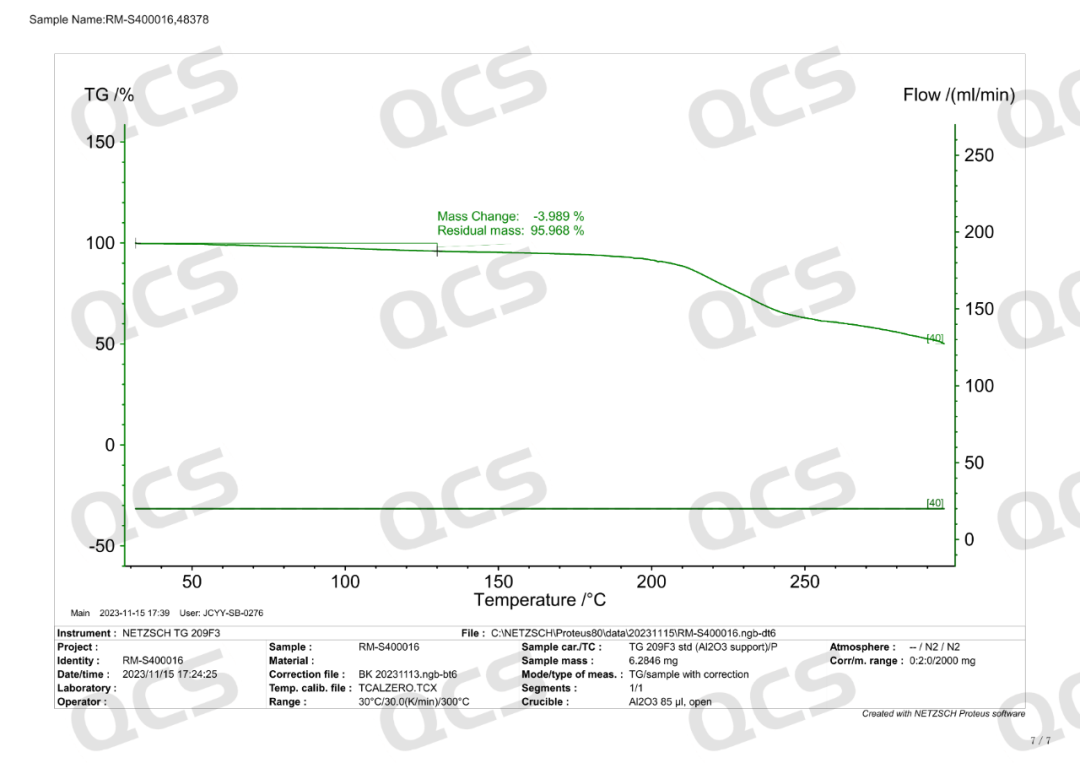
Semaglutide-D8 (CAT No.: RM-S400016) synthesized a deuterated derivative of semaglutide from deuterated valine with high isotopic purity. The purity of this product was determined to be 96% by HPLC, and confirmed to be trifluoroacetate by nuclear magnetic resonance hydrogen spectrum and fluorine spectrum. Mass spectrometry confirmed that it has extremely high isotopic purity. The product related testing report is shown in the following figure:
04 Summary
The QCS Standard Material Research and Development Center has achieved mass production of this product, which has good physical and chemical properties and is easy for researchers to use. The product is currently available in stock packaging of 1mg, 5mg, and 10mg. Please feel free to inquire.
01 Introduction
As winter fades away and the arrival of summer approaches, the lightweight clothing fails to conceal the restless human form. Recently, Elon Musk, CEO of Tesla, a company renowned for its innovative transportation solutions, has also joined the ranks of those seeking weight loss through pharmaceutical means. He has taken personally the newly trending slimming drug, Semaglutide.

02 Introduction to Semaglutide

According to the different causes of diabetes, the World Health Organization (WHO) divides diabetes into four types: Type 1 diabetes; Type 2 diabetes; Pregnancy diabetes; Special type diabetes, of which Type 2 diabetes is the most common type. For the treatment of Type 2 diabetes, the main products of new therapeutic drugs in recent years are GLP-1, SGLT-2 inhibitor and DPP4 inhibitor.
SGLT-2 inhibitors and DPP4 inhibitors were relatively launched earlier. In terms of SGLT-2 inhibitors, Dapagliflozin, jointly developed by Bristol-Myers Squibb and AstraZeneca, was launched in the European Union in 2012 and in the United States in 2014. Canagliflozin, which was acquired by Mitsubishi Pharmaceuticals and Johnson&Johnson's subsidiary of Johnson&Johnson, was launched in the United States in 2013. Empagliflozin, which is a partnership between Boehringer Ingelheim and Lilly, was approved by the FDA in 2014; In terms of DPP4 inhibitors, in 2006, Merck's original research of Sitagliptin was launched, Novartis Vildagliptin was launched in 2007, Saxagliptin, jointly developed by Bristol Myers Squibb and AstraZeneca, was launched in 2009, Takeda Alogliptin was launched in 2010, and so on. Although in recent years, new drugs have been continuously approved, such as the first self-developed net drug in China, Hengrui Pharmaceutical Innovation Drug, and SGLT2 inhibitor Henagliflozin, which was launched in 2021. However, GLP-1 drug is undoubtedly the most eye-catching star in recent years in the medical research and development massive free-for-all in the field of type 2 diabetes, and Smeaglutide is undoubtedly one of the trending products.
Semaglutide injection is a hit product of Novo Nordisk. According to Novo Nordisk's financial report, in the first half of 2023, Ozmmpic (trade name for slimming indications of Semaglutide injection) achieved revenue of 41.741 billion Danish kroner (1 Danish kroner is approximately equivalent to 1.05 RMB, QCS editor refers to the exchange rate from February 29, 2024), and Wegovy (trade name for slimming indications of Semaglutide injection) achieved revenue of 12.081 billion Danish kroner, totaling 53.822 billion Danish kroner, equivalent to 7.6 billion US dollars, with an expected total of 20 billion US dollars for the whole year. In the first half of 2023, Novo Nordisk's revenue in China was 8.928 billion Danish kroner, of which Semaglutide Injection (for blood sugar reduction) was 2.213 billion Danish kroner.
03 Independently developed stable isotope product of Semaglutide

Stable isotope markers of Semaglutide can be used for pharmacokinetic, safety, and tolerability studies. Novo Nordisk published two studies in 2018 on the pharmacokinetics, safety, and tolerability of smeglutide in subjects with liver injury or renal insufficiency. This stable isotope marker is used as an internal standard substance for plasma bioanalysis.
QCS Standard Material R&D Center has developed stable isotope products of Semaglutide-D8 and Semaglutide-D15 (CAT No.: RM-S400016 and RM-S400015) to meet the domestic pharmaceutical research and development needs. These stable isotopes can be applied in the research fields of drug bioavailability, drug distribution, biotransformation and excretion, drug interactions, drug stereoselectivity, etc., which is helpful for related pharmacokinetic studies.


This time, we will use the example of Semaglutide-D8 (CAT No.: RM-S400016), a deuterated valine modified analogue of semaglutide, to introduce QCS drug molecular stable isotope products.
Semaglutide-D8 (CAT No.: RM-S400016) synthesized a deuterated derivative of semaglutide from deuterated valine with high isotopic purity. The purity of this product was determined to be 96% by HPLC, and confirmed to be trifluoroacetate by nuclear magnetic resonance hydrogen spectrum and fluorine spectrum. Mass spectrometry confirmed that it has extremely high isotopic purity. The product related testing report is shown in the following figure:







04 Summary
The QCS Standard Material Research and Development Center has achieved mass production of this product, which has good physical and chemical properties and is easy for researchers to use. The product is currently available in stock packaging of 1mg, 5mg, and 10mg. Please feel free to inquire.
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号