Time:2025-03-28


Today we will share the study of the stability of specific impurities of paliperidone, a drug used to treat schizophrenia and schizoaffective disorder.
Experimental scheme
In this experiment, our center carried out solution stability studies on three specific impurities of paliperone according to the chromatographic conditions under "Paliperidone" variety item "ORGANIC IMPURITIES" of USP-NF2024 edition. The sample numbers and structural formulas used are shown in Figure 1 and Figure 2 below:
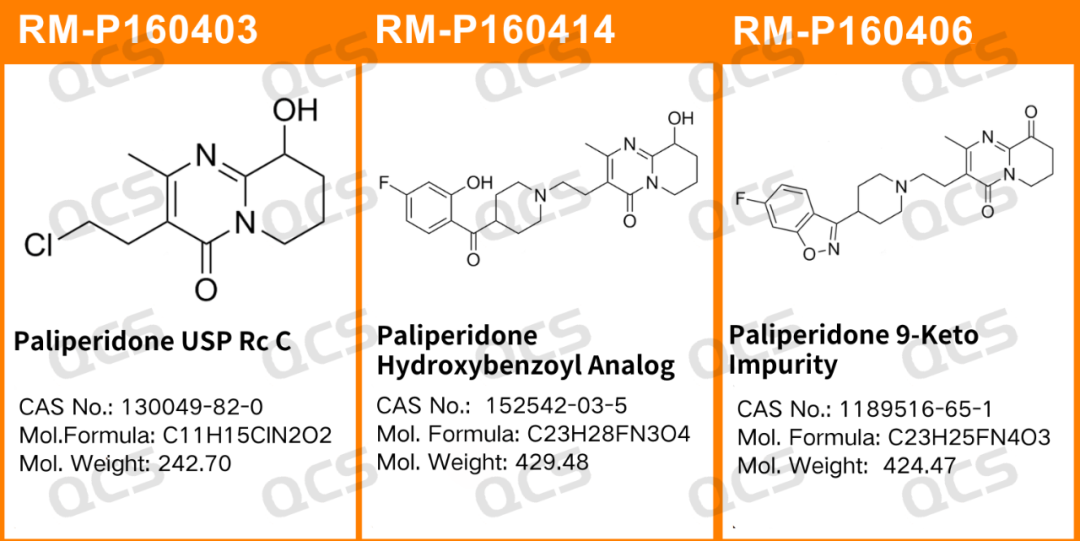
Figure 1: The impurity number and structure of the impurity used in this study
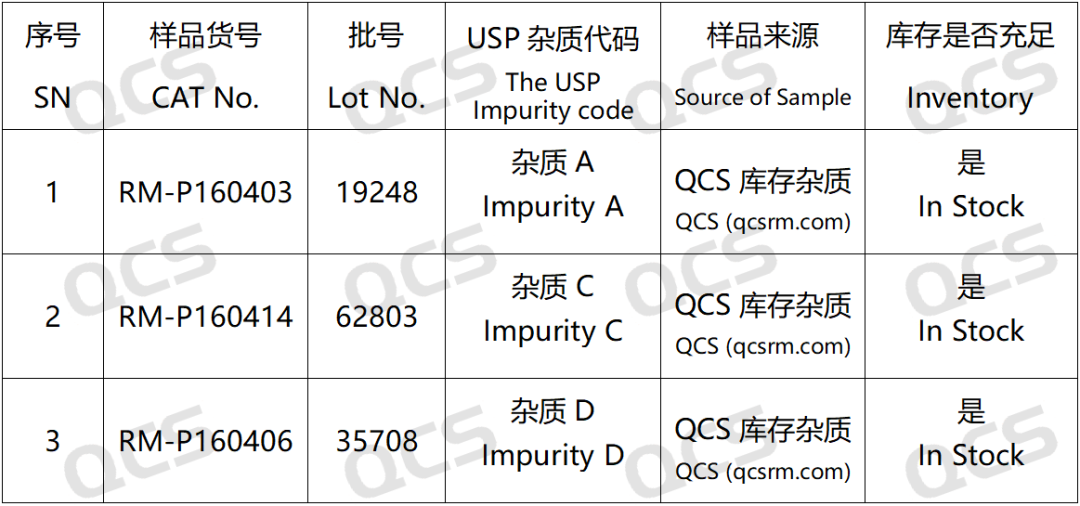
Figure 2: Standard inclusion of impurity codes and the correspondence between impurity numbers used in this study
In this experiment, the experimenter took appropriate amounts of RM-P160403 (USP impurity A; Paliperidone USP Rc C; CAS No:130049-82-0), RM-P160406(USP) and impurity D; Paliperidone 9-Keto Impurity; CAS No:1189516-65-1), as well as RM-P160414 (USP impurity C; Paliperidone Hydroxybenzoyl Analog; CAS No:152542-03-5)), and placed them in acidic, neutral, and alkaline solutions. The samples were left at room temperature and pressure for 0, 3, 6, 12, and 24 hours, respectively. Chromatographic conditions were then followed according to the "Paliperidone" section under "Item ORGANIC IMPURITIES" of USP-NF2024 edition for detection. The changes in the main peak area of the chromatogram over time were observed to determine the stability of the sample solution.
Experiment conclusion
After testing, it was found that the main peak area of sample RM-P160403 (USP impurity A) changed significantly over 24 hours when stored in acidic, neutral, and alkaline solutions, with relative standard deviations all exceeding 2.0%. Therefore, it can be concluded that this sample is unstable during storage for 24 hours in acidic, neutral, and alkaline solutions, degrading continuously as the storage time extends. The main peak area data for each detection point of sample RM-P160403 (USP impurity A) under various pH conditions are as follows:
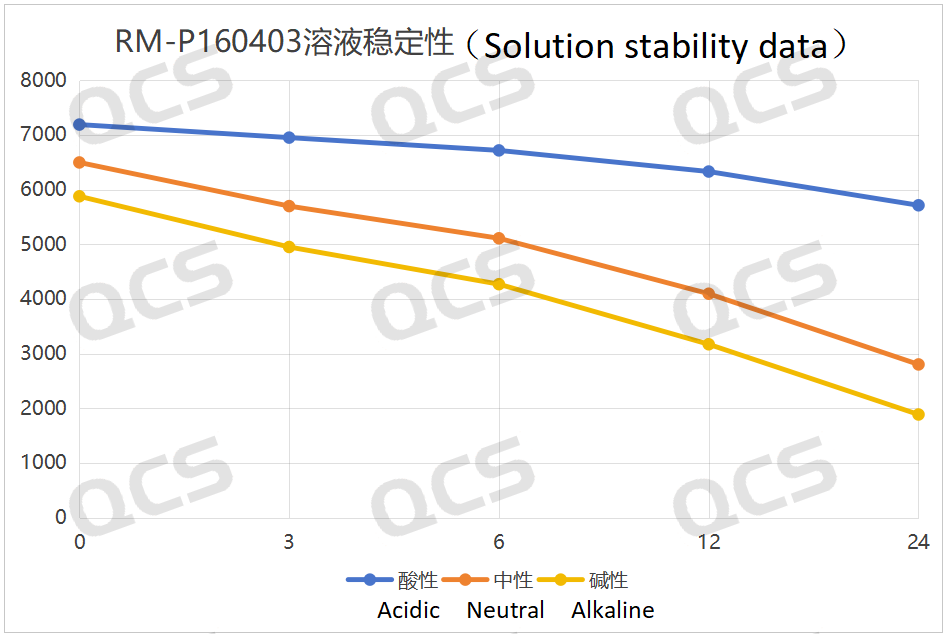
总折线图
Figure 3: Summary of the solution stability data of sample RM-P160403 (USP impurity A) after 24 hours in acidic, neutral and alkaline solutions
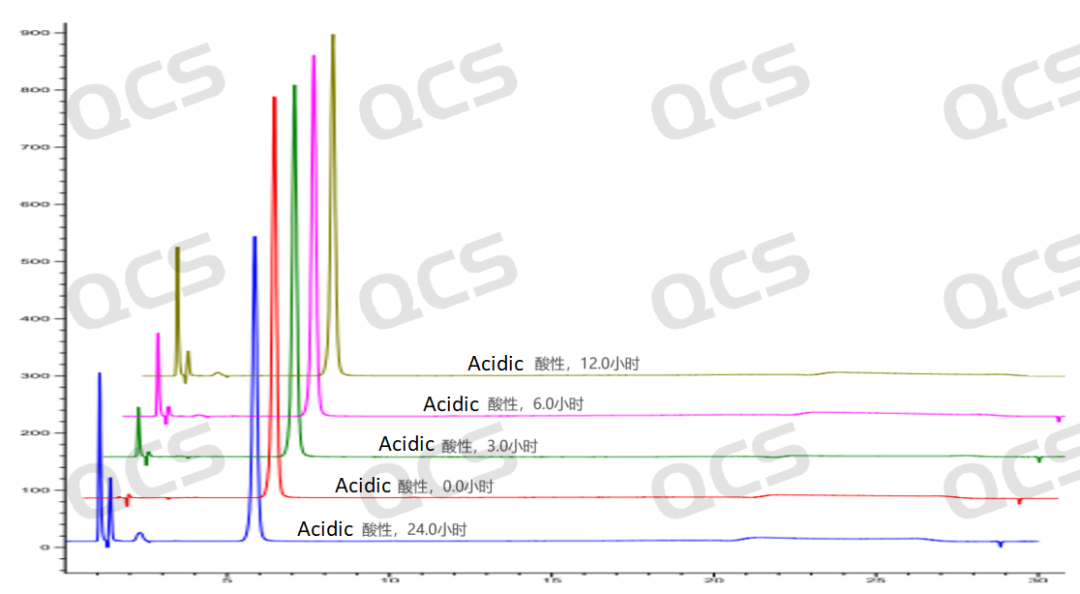
Figure 4: Stereoscopic comparison of acid solution stability data of sample RM-P160403 (USP impurity A)
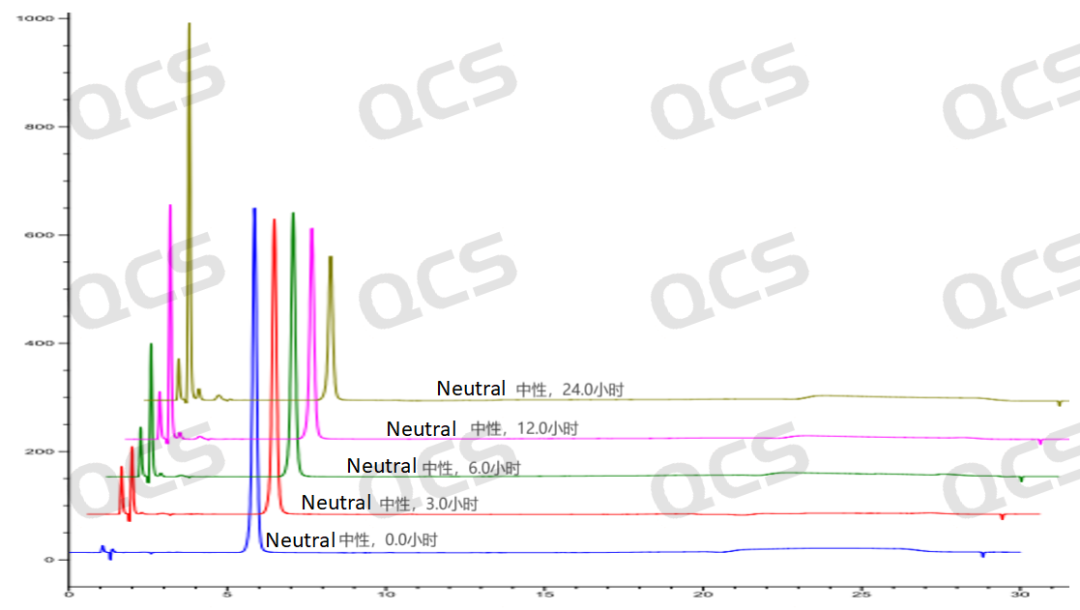
Figure 5: Stereoscopic comparison of the stability data of neutral solution of sample RM-P160403 (USP impurity A)
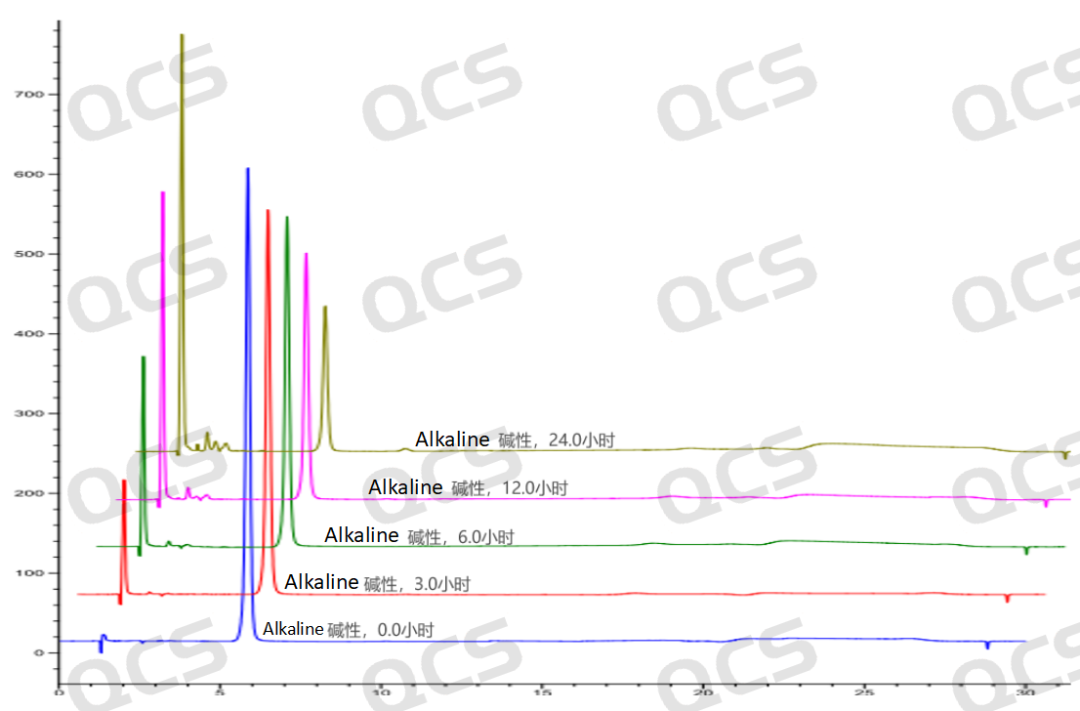
Figure 6: Stereoscopic comparison of the stability data of alkaline solution of sample RM-P160403 (USP impurity A)
After testing, it was found that the main peak area of sample RM-P160406 (USP impurity D) did not change significantly during storage in acidic and neutral solutions for 24 hours, with relative standard deviations all less than 2.0%. Therefore, it can be concluded that the sample is stable when stored in acidic and neutral solutions for 24 hours. However, the main peak area of the sample changed significantly during storage in alkaline solutions for 24 hours, with relative standard deviations greater than 2.0%. Thus, it can be concluded that the sample is unstable when stored in alkaline solutions for 24 hours and will degrade continuously over time. The main peak area data at each detection point for sample RM-P160406 (USP impurity D) under various pH conditions are as follows:
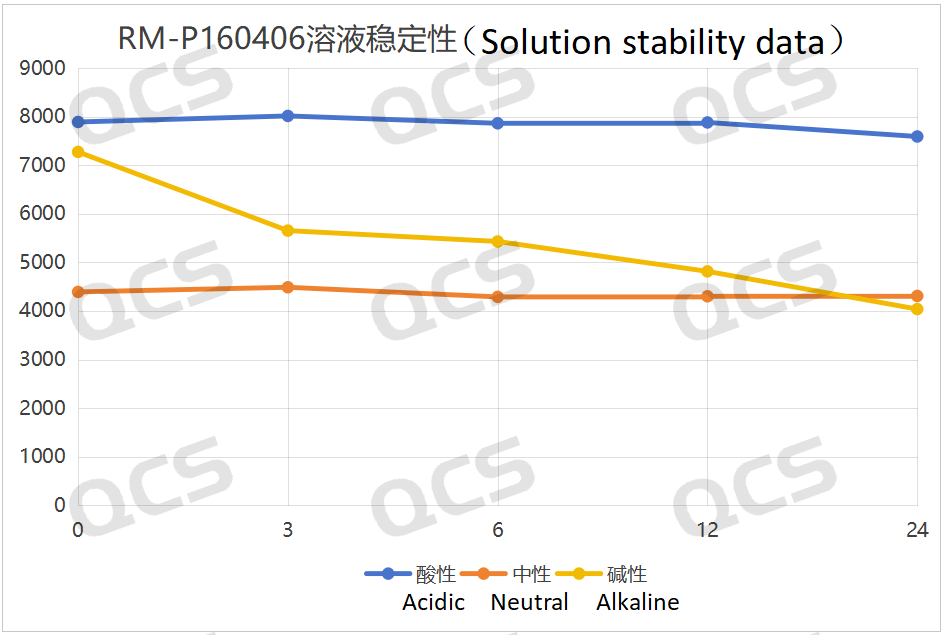
Figure 7: Summary of the solution stability data of sample RM-P160406 (USP impurity D) after 24 hours in acidic, neutral and alkaline solutions
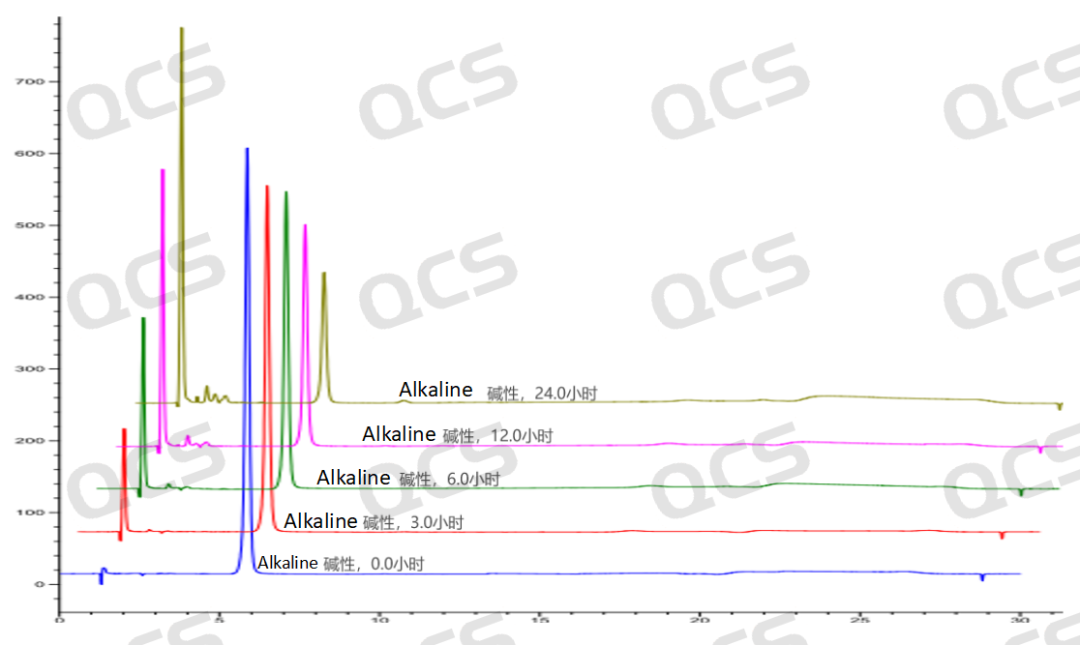
Figure 8: Stereoscopic comparison of the stability data of alkaline solution of sample RM-P160406 (USP impurity D)
After testing, it was found that the main peak area of sample RM-P160414 (USP impurity C) did not change significantly during storage in acidic and neutral solutions for 24 hours, with relative standard deviations all less than 2.0%. Therefore, it can be concluded that the sample remains stable during storage in both acidic and neutral solutions for 24 hours. The main peak area of the sample did not change significantly during storage in alkaline solutions for 6 hours, but after 6 hours, the sample experienced a slow degradation as time extended. The main peak area data at each detection point for sample RM-P160414 (USP impurity C) under various pH conditions are as follows:
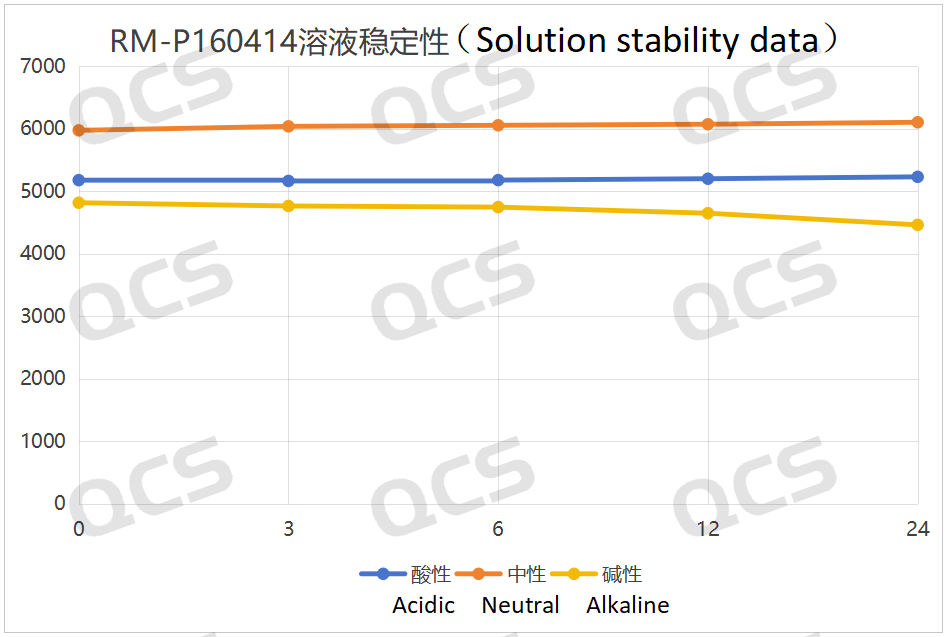
Figure 9: Summary of the solution stability data of sample RM-P160414 (USP impurity C) after 24 hours in acidic, neutral and alkaline solutions
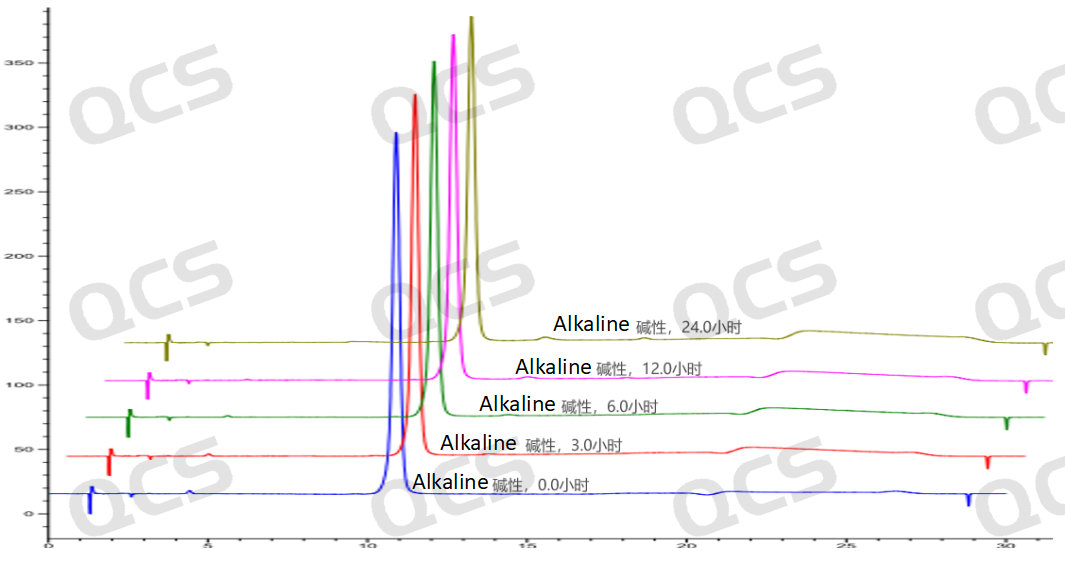
Figure 10: Stereoscopic comparison of stability data of alkaline solution of sample RM-P160414 (USP impurity C)
summary
In summary, through this experiment, we found that the sample RM-P160403 (USP impurity A) is unstable in acidic, neutral, and alkaline solutions. Therefore, customers should prepare and test the sample RM-P160403 (USP impurity A) immediately. The samples RM-P160406 (USP impurity D) and RM-P160414 (USP impurity C) are stable in acidic and neutral solutions but degrade over time with prolonged storage in alkaline solutions. Among them, the degradation rate of sample RM-P160406 (USP impurity D) is faster, while that of sample RM-P160414 (USP impurity C) is slower. Therefore, when testing the samples RM-P160406 (USP impurity D) and RM-P160414 (USP impurity C), customers should avoid using alkaline diluents and must not come into contact with alkali during use, storage, and transportation. If customers need information on the stability of these three samples, they can consult our company.




Today we will share the study of the stability of specific impurities of paliperidone, a drug used to treat schizophrenia and schizoaffective disorder.
Experimental scheme
In this experiment, our center carried out solution stability studies on three specific impurities of paliperone according to the chromatographic conditions under "Paliperidone" variety item "ORGANIC IMPURITIES" of USP-NF2024 edition. The sample numbers and structural formulas used are shown in Figure 1 and Figure 2 below:

Figure 1: The impurity number and structure of the impurity used in this study

Figure 2: Standard inclusion of impurity codes and the correspondence between impurity numbers used in this study
In this experiment, the experimenter took appropriate amounts of RM-P160403 (USP impurity A; Paliperidone USP Rc C; CAS No:130049-82-0), RM-P160406(USP) and impurity D; Paliperidone 9-Keto Impurity; CAS No:1189516-65-1), as well as RM-P160414 (USP impurity C; Paliperidone Hydroxybenzoyl Analog; CAS No:152542-03-5)), and placed them in acidic, neutral, and alkaline solutions. The samples were left at room temperature and pressure for 0, 3, 6, 12, and 24 hours, respectively. Chromatographic conditions were then followed according to the "Paliperidone" section under "Item ORGANIC IMPURITIES" of USP-NF2024 edition for detection. The changes in the main peak area of the chromatogram over time were observed to determine the stability of the sample solution.
Experiment conclusion
After testing, it was found that the main peak area of sample RM-P160403 (USP impurity A) changed significantly over 24 hours when stored in acidic, neutral, and alkaline solutions, with relative standard deviations all exceeding 2.0%. Therefore, it can be concluded that this sample is unstable during storage for 24 hours in acidic, neutral, and alkaline solutions, degrading continuously as the storage time extends. The main peak area data for each detection point of sample RM-P160403 (USP impurity A) under various pH conditions are as follows:

总折线图
Figure 3: Summary of the solution stability data of sample RM-P160403 (USP impurity A) after 24 hours in acidic, neutral and alkaline solutions

Figure 4: Stereoscopic comparison of acid solution stability data of sample RM-P160403 (USP impurity A)

Figure 5: Stereoscopic comparison of the stability data of neutral solution of sample RM-P160403 (USP impurity A)

Figure 6: Stereoscopic comparison of the stability data of alkaline solution of sample RM-P160403 (USP impurity A)
After testing, it was found that the main peak area of sample RM-P160406 (USP impurity D) did not change significantly during storage in acidic and neutral solutions for 24 hours, with relative standard deviations all less than 2.0%. Therefore, it can be concluded that the sample is stable when stored in acidic and neutral solutions for 24 hours. However, the main peak area of the sample changed significantly during storage in alkaline solutions for 24 hours, with relative standard deviations greater than 2.0%. Thus, it can be concluded that the sample is unstable when stored in alkaline solutions for 24 hours and will degrade continuously over time. The main peak area data at each detection point for sample RM-P160406 (USP impurity D) under various pH conditions are as follows:

Figure 7: Summary of the solution stability data of sample RM-P160406 (USP impurity D) after 24 hours in acidic, neutral and alkaline solutions

Figure 8: Stereoscopic comparison of the stability data of alkaline solution of sample RM-P160406 (USP impurity D)
After testing, it was found that the main peak area of sample RM-P160414 (USP impurity C) did not change significantly during storage in acidic and neutral solutions for 24 hours, with relative standard deviations all less than 2.0%. Therefore, it can be concluded that the sample remains stable during storage in both acidic and neutral solutions for 24 hours. The main peak area of the sample did not change significantly during storage in alkaline solutions for 6 hours, but after 6 hours, the sample experienced a slow degradation as time extended. The main peak area data at each detection point for sample RM-P160414 (USP impurity C) under various pH conditions are as follows:

Figure 9: Summary of the solution stability data of sample RM-P160414 (USP impurity C) after 24 hours in acidic, neutral and alkaline solutions

Figure 10: Stereoscopic comparison of stability data of alkaline solution of sample RM-P160414 (USP impurity C)
summary
In summary, through this experiment, we found that the sample RM-P160403 (USP impurity A) is unstable in acidic, neutral, and alkaline solutions. Therefore, customers should prepare and test the sample RM-P160403 (USP impurity A) immediately. The samples RM-P160406 (USP impurity D) and RM-P160414 (USP impurity C) are stable in acidic and neutral solutions but degrade over time with prolonged storage in alkaline solutions. Among them, the degradation rate of sample RM-P160406 (USP impurity D) is faster, while that of sample RM-P160414 (USP impurity C) is slower. Therefore, when testing the samples RM-P160406 (USP impurity D) and RM-P160414 (USP impurity C), customers should avoid using alkaline diluents and must not come into contact with alkali during use, storage, and transportation. If customers need information on the stability of these three samples, they can consult our company.


Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号