Time:2024-03-28
Lactulose is an artificially synthesized non absorbable disaccharide that does not exist in nature and is not absorbed in the small intestine. It is a commonly used clinical laxative that can be used for chronic or habitual constipation to regulate the physiological rhythm of the colon; It can also be used to treat hepatic encephalopathy and reduce blood ammonia.
01 Introduction
Today we will share a research on impurities related to a popular research and development product, lactulose oral liquid. Lactulose oral liquid is mainly used for the treatment of constipation, and it is said that there are currently no competitors in China. According to menet.com.cn, in 2021, Lactulose oral solution ranked first among the chemical products used to treat constipation. Menet data shows, the sales revenue of this product in China's three major terminals and six major markets has been increasing year by year in recent years. In 2021, it exceeded 1.8 billion yuan, a year-on-year increase of 23.08%.
02 Introduction to Impurity F in Lactulose EP
Lactulose Oral Solution is a brown yellow clear viscous liquid. Used to treat hyperammonemia and diseases caused by elevated blood ammonia levels; Used to treat chronic functional constipation.
At present, the QCS official website has a total of 22 impurities in Lactulose (scan the QR code at the end of the article to view the list of all impurities), among which the EP Impurity F in Lactulose poses certain challenges in directional synthesis due to its unique structure.Our center adopts directional synthesis method, and a total of eight steps chemical reactions can efficiently prepare Lactulose EP Impurity F standard (QCS article number: RM-L010316, structural information is shown in Figure 1).
For this product, our center has conducted research based on the registration standard for imported raw materials of “Concentrated solution of lactulose” (standard number JX20170210) and literature such as the European Pharmacopoeia. A qualitative positioning comparison study was conducted between the European Pharmacopoeia peak identification standard and the QCS produced Lactulose Impurity F standard, effectively confirming the applicability of the QCS Lactulose impurity F standard. This article will share information on the physical and chemical properties, chromatographic results, and characteristics of Lactulose EP impurity F.
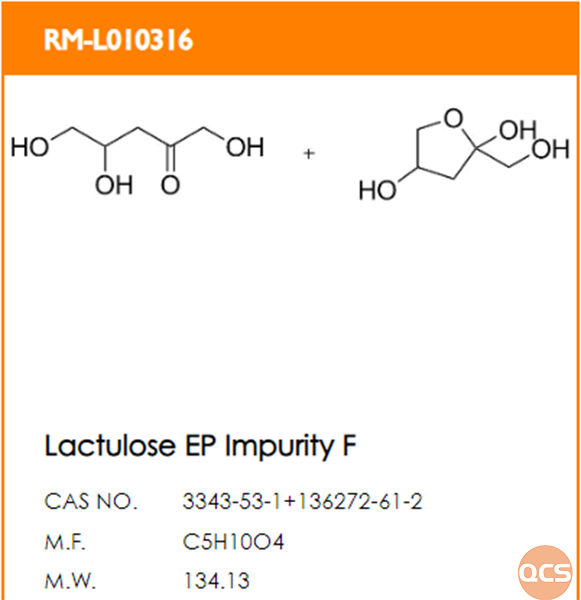
Figure 1: Structural information of Lactulose EP impurity F
03 Research on Lactulose Related Substances
Research on Lactulose related substances can refer to the raw material import registration standard (standard number JX20170210) of "Concentrated solution of lactulose". The relevant substance method of this import registration standard uses aminopropyl bonded silica gel as the stationary phase and a parallax refractive detector for determination. The specific conditions and the limits of various impurities in the standard are shown in Figure 2 and Figure 3.

Figure 2: Substance methods related to the registration standards for imported raw materials
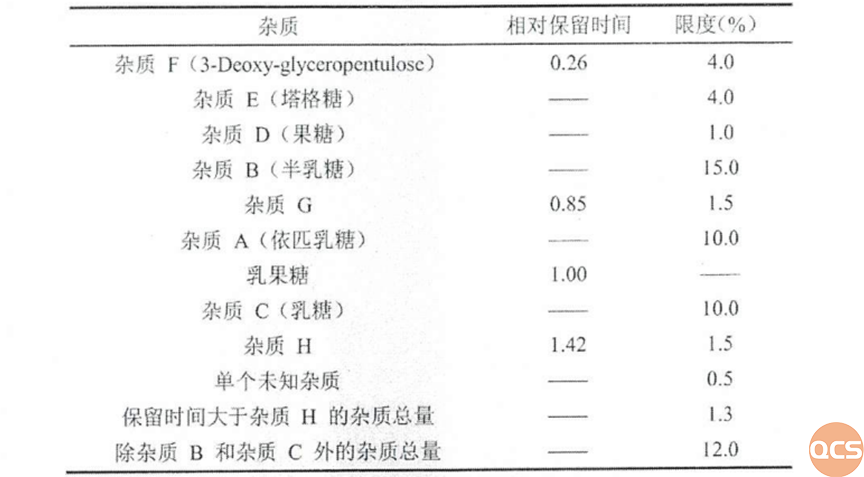
Figure 3: Limits of impurities in the registration standards for raw material imports
04 Comparative study on qualitative localization of Lactulose Peak Identification Reference and Lactulose Impurity F Standard
Due to the good separation effect of aminopropyl bonded silica gel stationary phase on carbohydrate substances, and the good sensitivity of the parallax refractive detector to this type of substance (see Figure 4 for peak identification CRS typical chromatogram). In order to verify the consistency between the Lactulose Impurity F produced by our center and the EP standard, the QCS R&D center conducted a series of qualitative positioning comparative studies using the European Pharmacopoeia (EP) standard Lactulose peak identification CRS cat#Y0001510 and the Lactulose Impurity F standard produced by QCS.
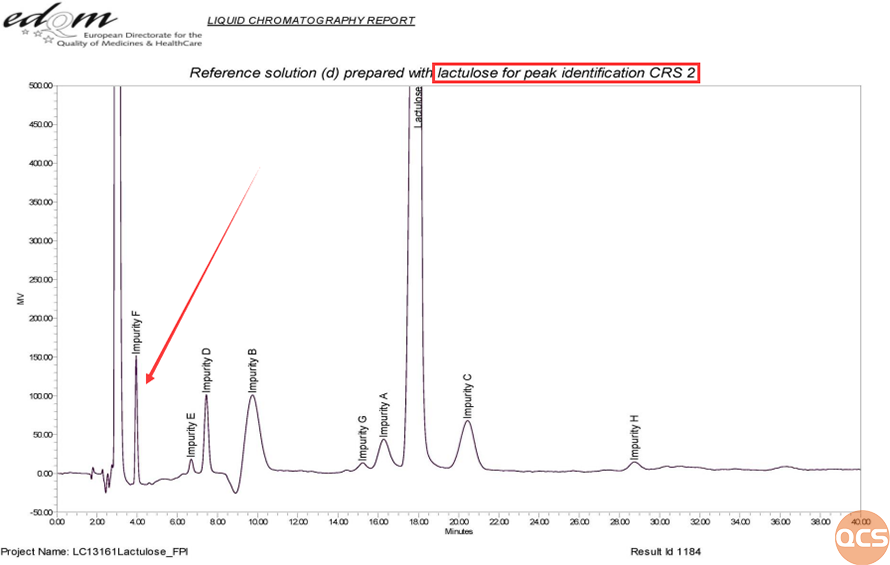
Figure 4: Typical chromatogram of peak identification CRS
Our center used similar chromatographic fillers as stationary phases and employed a parallax refractive detector as the detection method to establish a chromatographic detection environment for Lactulose Impurity F standard. Before conducting the detection of Lactulose Impurity F substance, our center first used the European Pharmacopoeia (EP) standard Lactose for peak identification CRS substance (cat#Y0001510) to validate our center's chromatographic conditions and reproduce the standard chromatographic results of CRS. The specific reproducibility data is shown in Figure 5:
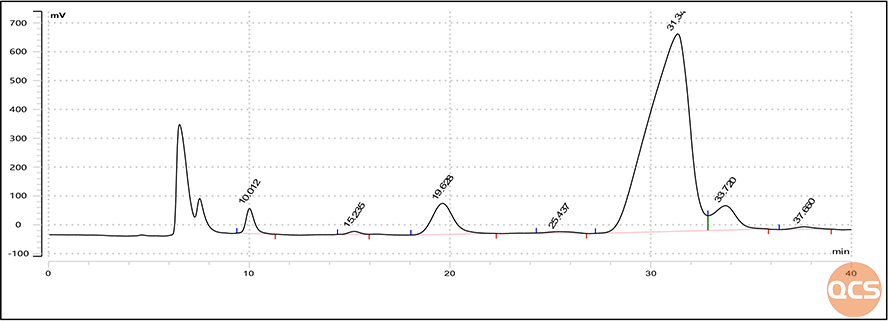
Figure 5: Peak identification reference QCS reproduction chart
From Figure 5, it can be seen that the various components in the Lactulose peak identification reference substance can be effectively separated using our central chromatographic method. The relative proportions and peak order of each component are consistent with the typical chromatogram of CRS, and can basically reproduce the typical chromatogram of the Lactulose peak identification reference substance in the European Pharmacopoeia (EP) standard substance. Subsequently, under this method, I injected Lactulose EP impurity F into the center to validate our product (see Figure 6 for the chromatographic results of our product).
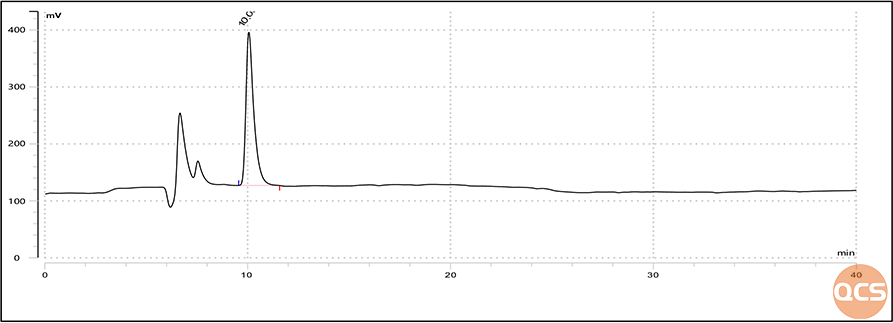
Figure 6: Injection chromatogram of EP impurity F in Lactulose produced by QCS
From the graph, it can be seen that the retention time of impurity F is consistent with that of impurity F in CRS. In order to verify the accuracy of impurity F again, we added impurity F to the European Pharmacopoeia (EP) standard lactulose peak identification reference sample solution, and the mixed injection data obtained are shown in Figure 7:
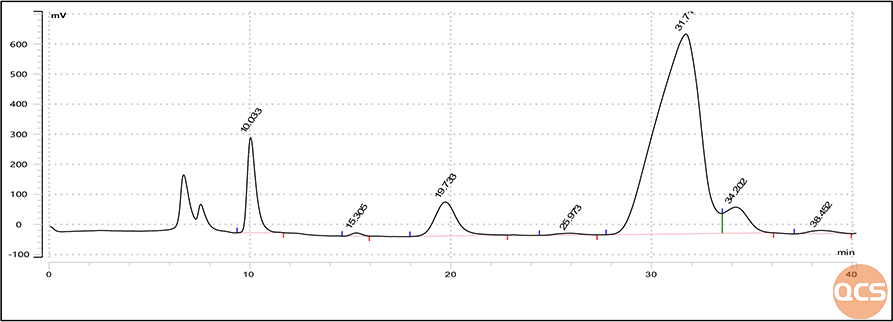
Figure 7: Peak identification mixed sample chromatogram of QCS Lactulose EP Impurity F and EP
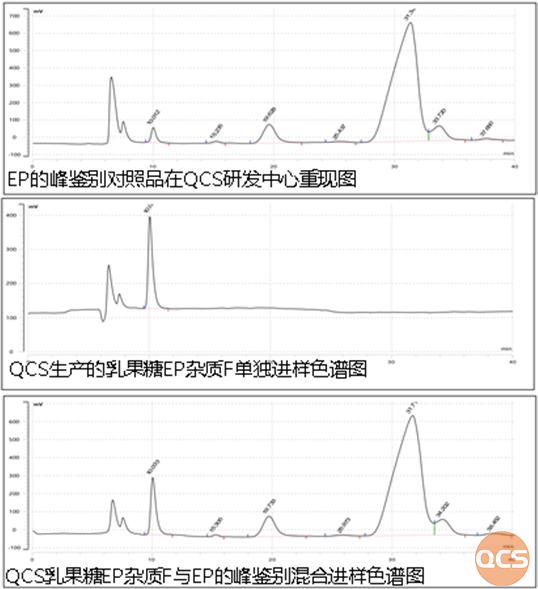
Figure 8: Comparison of peak identification chromatographic results between QCS Lactulose EP Impurity F and EP
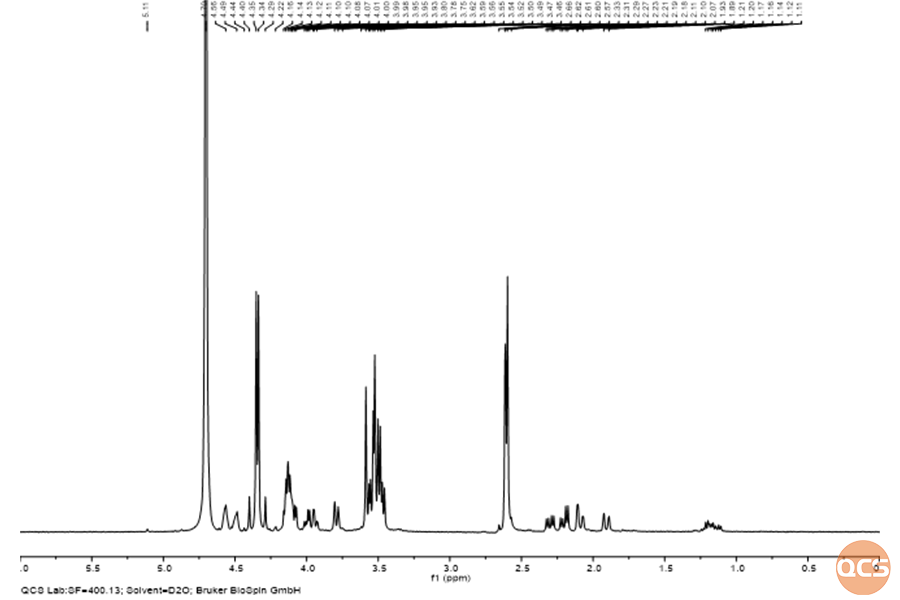
Figure 9: H-NMR spectrum of EP impurity F in Lactulose produced by QCS
From the comparison of data before and after, it can be seen that the Impurity F of Lactulose EP produced by QCS shows a single peak, and its retention time is consistent with the retention time of Impurity F in the peak identification sample. Meanwhile, in the mixed injection results of QCS Lactulose EP impurity F and EP peak identification standard, the addition of QCS Lactulose EP Impurity F did not produce new impurity peaks, only the peak area of impurity F changed significantly (area increased from 2000 to 9000), and there was no significant change in the peak area of other impurities.
The above results indicate that the Lactulose EP Impurity F produced by QCS has good applicability and high chromatographic purity (purity close to 100%). The product exhibits a single peak under import registration standards or European Pharmacopoeia conditions. Double peaks are only theoretically possible and do not hold true in this chromatographic system. As long as the purity meets the requirements, the product should be a single peak under these chromatographic conditions.
05 Stability testing experiment of Lactulose EP Impurity F
In order to better characterize the impurity F of Lactulose EP, the QCS R&D center conducted H-NMR spectroscopy testing on the product (see Figure 9 for the results). At the same time, based on the non-destructive testing provided by NMR, the QCS R&D center conducted a stability test experiment on Lactulose EP Impurity F under heavy water conditions for several days (the stability test results are shown in Figure 10).
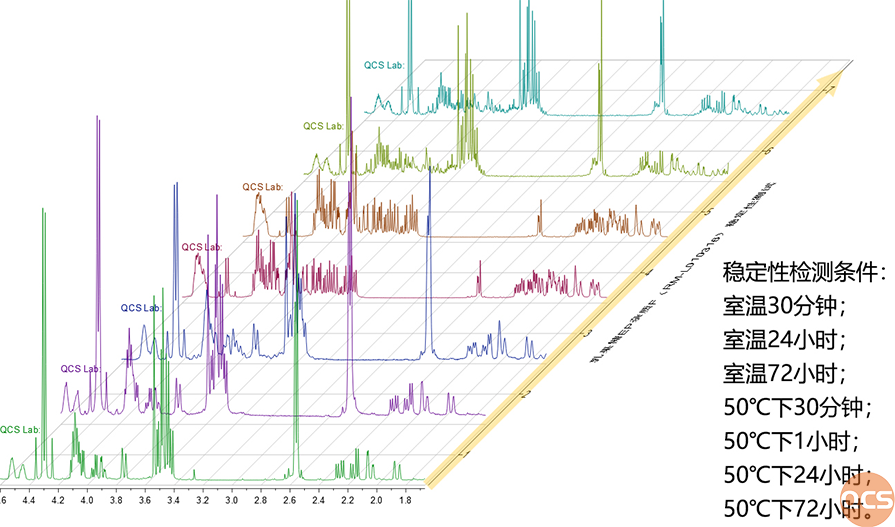
Figure 10: Stability monitoring results of Lactulose EP impurity F based on NMR method
Through stability testing of products under different temperature and time conditions, it can be seen that the impurity F of Lactulose EP has a certain sensitivity to temperature, and the product will undergo significant degradation under long-term room temperature or short-term high temperature conditions. Therefore, the QCS R&D center reminds you to control the temperature during the use of Lactulose EP Impurity F to prevent product degradation caused by heating, which may affect your use.
06 Summary
Our center adopts directional synthesis method, and a total of eight steps chemical reactions can efficiently prepare Lactulose EP Impurity F standard (QCS article number: RM-L010316, structural information is shown in Figure 1). This product is a colorless oily liquid and is highly soluble in water or acetonitrile. Due to the challenge of detecting sugar structures, our center referred to the registration standard for raw material import of “Concentrated solution of lactulose” (standard number JX20170210) and conducted a qualitative localization comparison study using the European Pharmacopoeia peak identification and the Lactulose Impurity F standard produced by QCS. The liquid chromatography results were qualified. Based on the stability monitoring results of Lactose EP Impurity F using NMR method, it is recommended to pay attention to low-temperature storage of this product.
Researcher Ms. Qiu from the Preparation and Separation Department of QCS R&D Center has made great contributions to the purification and separation of this project. From the testing of sample analysis and detection methods to the development of chromatographic purification processes, Researcher Ms. Qiu screened over ten types of packing stationary phases and chromatographic methods, and compiled dozens of pages of the “QCS R&D Center Lactulose Related Substance F Purification Preparation Report”. She conducted systematic research on product stability, product purity testing, and storage/transportation methods. We hope this research data can be helpful to customers who use Lactulose related substance F.

Long press the recognition QR code to view a list of all impurities!

Lactulose is an artificially synthesized non absorbable disaccharide that does not exist in nature and is not absorbed in the small intestine. It is a commonly used clinical laxative that can be used for chronic or habitual constipation to regulate the physiological rhythm of the colon; It can also be used to treat hepatic encephalopathy and reduce blood ammonia.
01 Introduction
Today we will share a research on impurities related to a popular research and development product, lactulose oral liquid. Lactulose oral liquid is mainly used for the treatment of constipation, and it is said that there are currently no competitors in China. According to menet.com.cn, in 2021, Lactulose oral solution ranked first among the chemical products used to treat constipation. Menet data shows, the sales revenue of this product in China's three major terminals and six major markets has been increasing year by year in recent years. In 2021, it exceeded 1.8 billion yuan, a year-on-year increase of 23.08%.
02 Introduction to Impurity F in Lactulose EP
Lactulose Oral Solution is a brown yellow clear viscous liquid. Used to treat hyperammonemia and diseases caused by elevated blood ammonia levels; Used to treat chronic functional constipation.
At present, the QCS official website has a total of 22 impurities in Lactulose (scan the QR code at the end of the article to view the list of all impurities), among which the EP Impurity F in Lactulose poses certain challenges in directional synthesis due to its unique structure.Our center adopts directional synthesis method, and a total of eight steps chemical reactions can efficiently prepare Lactulose EP Impurity F standard (QCS article number: RM-L010316, structural information is shown in Figure 1).
For this product, our center has conducted research based on the registration standard for imported raw materials of “Concentrated solution of lactulose” (standard number JX20170210) and literature such as the European Pharmacopoeia. A qualitative positioning comparison study was conducted between the European Pharmacopoeia peak identification standard and the QCS produced Lactulose Impurity F standard, effectively confirming the applicability of the QCS Lactulose impurity F standard. This article will share information on the physical and chemical properties, chromatographic results, and characteristics of Lactulose EP impurity F.

Figure 1: Structural information of Lactulose EP impurity F
03 Research on Lactulose Related Substances
Research on Lactulose related substances can refer to the raw material import registration standard (standard number JX20170210) of "Concentrated solution of lactulose". The relevant substance method of this import registration standard uses aminopropyl bonded silica gel as the stationary phase and a parallax refractive detector for determination. The specific conditions and the limits of various impurities in the standard are shown in Figure 2 and Figure 3.

Figure 2: Substance methods related to the registration standards for imported raw materials

Figure 3: Limits of impurities in the registration standards for raw material imports
04 Comparative study on qualitative localization of Lactulose Peak Identification Reference and Lactulose Impurity F Standard
Due to the good separation effect of aminopropyl bonded silica gel stationary phase on carbohydrate substances, and the good sensitivity of the parallax refractive detector to this type of substance (see Figure 4 for peak identification CRS typical chromatogram). In order to verify the consistency between the Lactulose Impurity F produced by our center and the EP standard, the QCS R&D center conducted a series of qualitative positioning comparative studies using the European Pharmacopoeia (EP) standard Lactulose peak identification CRS cat#Y0001510 and the Lactulose Impurity F standard produced by QCS.

Figure 4: Typical chromatogram of peak identification CRS
Our center used similar chromatographic fillers as stationary phases and employed a parallax refractive detector as the detection method to establish a chromatographic detection environment for Lactulose Impurity F standard. Before conducting the detection of Lactulose Impurity F substance, our center first used the European Pharmacopoeia (EP) standard Lactose for peak identification CRS substance (cat#Y0001510) to validate our center's chromatographic conditions and reproduce the standard chromatographic results of CRS. The specific reproducibility data is shown in Figure 5:

Figure 5: Peak identification reference QCS reproduction chart
From Figure 5, it can be seen that the various components in the Lactulose peak identification reference substance can be effectively separated using our central chromatographic method. The relative proportions and peak order of each component are consistent with the typical chromatogram of CRS, and can basically reproduce the typical chromatogram of the Lactulose peak identification reference substance in the European Pharmacopoeia (EP) standard substance. Subsequently, under this method, I injected Lactulose EP impurity F into the center to validate our product (see Figure 6 for the chromatographic results of our product).

Figure 6: Injection chromatogram of EP impurity F in Lactulose produced by QCS
From the graph, it can be seen that the retention time of impurity F is consistent with that of impurity F in CRS. In order to verify the accuracy of impurity F again, we added impurity F to the European Pharmacopoeia (EP) standard lactulose peak identification reference sample solution, and the mixed injection data obtained are shown in Figure 7:

Figure 7: Peak identification mixed sample chromatogram of QCS Lactulose EP Impurity F and EP

Figure 8: Comparison of peak identification chromatographic results between QCS Lactulose EP Impurity F and EP

Figure 9: H-NMR spectrum of EP impurity F in Lactulose produced by QCS
From the comparison of data before and after, it can be seen that the Impurity F of Lactulose EP produced by QCS shows a single peak, and its retention time is consistent with the retention time of Impurity F in the peak identification sample. Meanwhile, in the mixed injection results of QCS Lactulose EP impurity F and EP peak identification standard, the addition of QCS Lactulose EP Impurity F did not produce new impurity peaks, only the peak area of impurity F changed significantly (area increased from 2000 to 9000), and there was no significant change in the peak area of other impurities.
The above results indicate that the Lactulose EP Impurity F produced by QCS has good applicability and high chromatographic purity (purity close to 100%). The product exhibits a single peak under import registration standards or European Pharmacopoeia conditions. Double peaks are only theoretically possible and do not hold true in this chromatographic system. As long as the purity meets the requirements, the product should be a single peak under these chromatographic conditions.
05 Stability testing experiment of Lactulose EP Impurity F
In order to better characterize the impurity F of Lactulose EP, the QCS R&D center conducted H-NMR spectroscopy testing on the product (see Figure 9 for the results). At the same time, based on the non-destructive testing provided by NMR, the QCS R&D center conducted a stability test experiment on Lactulose EP Impurity F under heavy water conditions for several days (the stability test results are shown in Figure 10).

Figure 10: Stability monitoring results of Lactulose EP impurity F based on NMR method
Through stability testing of products under different temperature and time conditions, it can be seen that the impurity F of Lactulose EP has a certain sensitivity to temperature, and the product will undergo significant degradation under long-term room temperature or short-term high temperature conditions. Therefore, the QCS R&D center reminds you to control the temperature during the use of Lactulose EP Impurity F to prevent product degradation caused by heating, which may affect your use.
06 Summary
Our center adopts directional synthesis method, and a total of eight steps chemical reactions can efficiently prepare Lactulose EP Impurity F standard (QCS article number: RM-L010316, structural information is shown in Figure 1). This product is a colorless oily liquid and is highly soluble in water or acetonitrile. Due to the challenge of detecting sugar structures, our center referred to the registration standard for raw material import of “Concentrated solution of lactulose” (standard number JX20170210) and conducted a qualitative localization comparison study using the European Pharmacopoeia peak identification and the Lactulose Impurity F standard produced by QCS. The liquid chromatography results were qualified. Based on the stability monitoring results of Lactose EP Impurity F using NMR method, it is recommended to pay attention to low-temperature storage of this product.
Researcher Ms. Qiu from the Preparation and Separation Department of QCS R&D Center has made great contributions to the purification and separation of this project. From the testing of sample analysis and detection methods to the development of chromatographic purification processes, Researcher Ms. Qiu screened over ten types of packing stationary phases and chromatographic methods, and compiled dozens of pages of the “QCS R&D Center Lactulose Related Substance F Purification Preparation Report”. She conducted systematic research on product stability, product purity testing, and storage/transportation methods. We hope this research data can be helpful to customers who use Lactulose related substance F.

Long press the recognition QR code to view a list of all impurities!

Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
Join Our Email List
Subscribe to receive updates on new
products, promotions and resources!
| ISO 17034:2016 |
| ISO 9001:2015 |

*All our products are for R&D.

*All our products are for R&D.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号