orders@qcsrm.com | +86 755-6685 3366 | 2851296953 | 13670046396
orders@qcsrm.com | +86 755-6685 3366 | 2851296953 | +86-13670046396
Tiempo tiempo:2025-10-31

Tacrolimus (FK506), a fermentation product isolated from Streptomyces, is a macrolide antibiotic and a potent new immunosuppressant. It exerts its immunosuppressive effect by inhibiting interleukin-2 (IL-2) release, thereby suppressing T lymphocyte activity. Its potency is 100 times greater than that of cyclosporine A (CsA).
In recent years, FK506, a first-line drug for liver and kidney transplantation, has been approved in 14 countries including Japan and the United States. Clinical trials demonstrate its efficacy in heart, lung, intestinal, and bone marrow transplants. Additionally, FK506 shows promising therapeutic effects in autoimmune diseases such as atopic dermatitis (AD), systemic lupus erythematosus (SLE), and autoimmune eye disorders.
I. Research Background
Tacrolimus is characterized by a 23-membered macrolide core with high conformational flexibility and tension, enabling multiple spatial configurations. Its oxygen-containing functional groups—esters, ethers, ketones, and hydroxyl groups—contribute to strong polarity and hydrogen bonding, while also creating complex intramolecular interactions. The molecule contains over ten asymmetric carbon atoms that form multiple stereochemical centers. The relative and absolute configurations of these chiral elements collectively determine its unique three-dimensional structure and biological activity.
In the third 2025 issue of "2025V3 | Stability Study of Tacrolimus (New Immunosuppressant) -Specific Impurities", we presented stability testing and separation results for Tacrolimus and its impurity RM-T031205 (EP Impurity I: Tacrolimus Diene/Tacrolimus EP Impurity I; CAS NO: 104987-16-8). However, Tacrolimus' complex structure makes the active pharmaceutical ingredient susceptible to degradation during production due to pH, temperature, humidity, and light exposure, resulting in over 100 impurities.
As one of our company's best-selling impurity series, the QCS Standard Material Research Center conducted studies on multiple tacrolimus impurities in accordance with the registration standards for tacrolimus cream. Four selected impurities are summarized below (structure shown in Figure 1).

Figure 1: Structure of tacrolimus and tacrolimus-related substances
II. Research on HPLC Method
After conducting NMR and MS qualitative analysis on tacrolimus impurities, our company performed liquid chromatography analysis according to the impurities method specified in the registration standard for Tacrolimus Cream. The test results under the established protocol are shown in Figures 2 and 4 below.
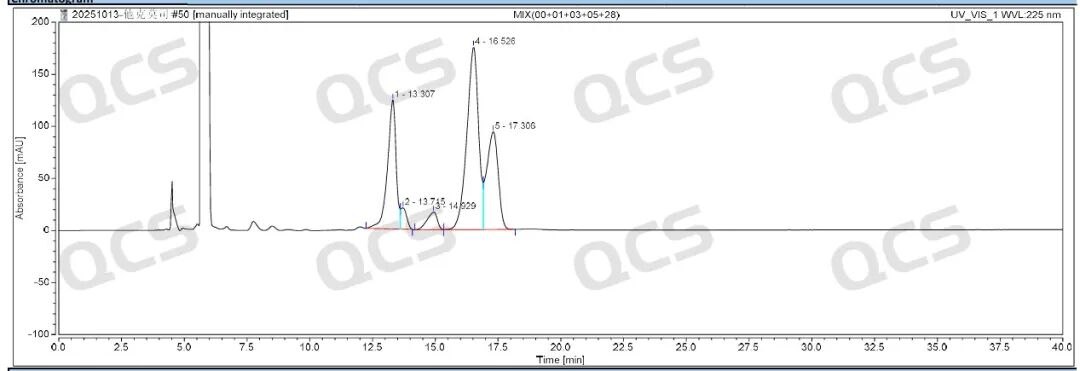
Figure 2: Tacrolimus impurity profile

Figure 3: Information on related substances in the registration criteria for tacrolimus cream

Figure 4: Relative retention times of impurities in tacrolimus
The results indicate that the relative retention times of all impurities are within the standard limits, demonstrating our company's successful implementation of central control and monitoring for the four tacrolimus-related substances.
3. Conclusion
Tacrolimus, as a vital immunosuppressant, plays a pivotal role in organ transplantation and autoimmune disease treatment. However, its production process faces numerous challenges, with impurity issues being particularly prominent, mainly involving poor product stability, complex synthesis, and diverse detection methods.
These research findings provide crucial technical support for the quality control and process optimization of tacrolimus, contributing to improved product quality and medication safety. Going forward, we will further advance related studies to develop more sophisticated impurity control strategies and analytical methods.
Through standardized methods, our company maintains strict quality control over tacrolimus impurities. As one of our best-selling impurity series, these impurities feature complete chromatographic profiles, high purity, competitive pricing, and customization options. For more information on related products and services, please feel free to inquire.
Figure 5: Tacrolimus assay data



Tacrolimus (FK506), a fermentation product isolated from Streptomyces, is a macrolide antibiotic and a potent new immunosuppressant. It exerts its immunosuppressive effect by inhibiting interleukin-2 (IL-2) release, thereby suppressing T lymphocyte activity. Its potency is 100 times greater than that of cyclosporine A (CsA).
In recent years, FK506, a first-line drug for liver and kidney transplantation, has been approved in 14 countries including Japan and the United States. Clinical trials demonstrate its efficacy in heart, lung, intestinal, and bone marrow transplants. Additionally, FK506 shows promising therapeutic effects in autoimmune diseases such as atopic dermatitis (AD), systemic lupus erythematosus (SLE), and autoimmune eye disorders.
I. Research Background
Tacrolimus is characterized by a 23-membered macrolide core with high conformational flexibility and tension, enabling multiple spatial configurations. Its oxygen-containing functional groups—esters, ethers, ketones, and hydroxyl groups—contribute to strong polarity and hydrogen bonding, while also creating complex intramolecular interactions. The molecule contains over ten asymmetric carbon atoms that form multiple stereochemical centers. The relative and absolute configurations of these chiral elements collectively determine its unique three-dimensional structure and biological activity.
In the third 2025 issue of "2025V3 | Stability Study of Tacrolimus (New Immunosuppressant) -Specific Impurities", we presented stability testing and separation results for Tacrolimus and its impurity RM-T031205 (EP Impurity I: Tacrolimus Diene/Tacrolimus EP Impurity I; CAS NO: 104987-16-8). However, Tacrolimus' complex structure makes the active pharmaceutical ingredient susceptible to degradation during production due to pH, temperature, humidity, and light exposure, resulting in over 100 impurities.
As one of our company's best-selling impurity series, the QCS Standard Material Research Center conducted studies on multiple tacrolimus impurities in accordance with the registration standards for tacrolimus cream. Four selected impurities are summarized below (structure shown in Figure 1).

Figure 1: Structure of tacrolimus and tacrolimus-related substances
II. Research on HPLC Method
After conducting NMR and MS qualitative analysis on tacrolimus impurities, our company performed liquid chromatography analysis according to the impurities method specified in the registration standard for Tacrolimus Cream. The test results under the established protocol are shown in Figures 2 and 4 below.

Figure 2: Tacrolimus impurity profile

Figure 3: Information on related substances in the registration criteria for tacrolimus cream

Figure 4: Relative retention times of impurities in tacrolimus
The results indicate that the relative retention times of all impurities are within the standard limits, demonstrating our company's successful implementation of central control and monitoring for the four tacrolimus-related substances.
3. Conclusion
Tacrolimus, as a vital immunosuppressant, plays a pivotal role in organ transplantation and autoimmune disease treatment. However, its production process faces numerous challenges, with impurity issues being particularly prominent, mainly involving poor product stability, complex synthesis, and diverse detection methods.
These research findings provide crucial technical support for the quality control and process optimization of tacrolimus, contributing to improved product quality and medication safety. Going forward, we will further advance related studies to develop more sophisticated impurity control strategies and analytical methods.
Through standardized methods, our company maintains strict quality control over tacrolimus impurities. As one of our best-selling impurity series, these impurities feature complete chromatographic profiles, high purity, competitive pricing, and customization options. For more information on related products and services, please feel free to inquire.

Figure 5: Tacrolimus assay data


Únase a nuestra lista de correo electrónico
Suscríbase para recibir actualizaciones sobre nuevos productos, promociones y recursos!
Únase a nuestra lista de correo electrónico
Suscríbase para recibir actualizaciones sobre nuevos productos, promociones y recursos!
| ISO 17034:2016 |
| ISO 9001:2015 |

*Todos los productos de esta empresa son solo para uso en investigación científica.
| Ejemplo de COA (Certificado de Análisis) |
| Certificado de Empresa de Alta Tecnología |
| ISO 17034:2016 |
| ISO 9001:2015 |

*Todos los productos de esta empresa son solo para uso en investigación científica.
Copyright © 2021-2024 QCSRM All rights reserved. 粤ICP备2023004355号
Copyright © 2021-2024 QCSRM All rights reserved.
粤ICP备2023004355号